Sodium (Na) and potassium (K) are elements with rich resources that arewidely spread in use around the world. If applied to the alkaline metal ion batteries, theycan ensure the sustainable, low-cost and stable growth of the large-scale energy storage system market. However, the relatively large radii of Na+ and K+ cause a low process of intercalation/ de-intercalation dynamics. Moreover, the electrode material bears large mechanical stress in the process of repeated intercalation/de-intercalation of alkali metal ions, which causes more serious volume change and structural damage, resulting in a rapidly reduced capacity. At present,many materialshave been developed byresearchers as theanodes for sodium-ion batteries(SIBs) andpotassium-ion batteries (KIBs). Among them, hard carbon materials composed of disordered graphite microcrystals show excellent electrochemical performance and have a great potential for development.

ZHANG Yingjie and LI Xue, Professors of the National and Local Joint Engineering Laboratory for Lithium-Ion Batteries and Materials Fabrication Technology, Faculty of Metallurgical and Energy Engineering, KUST,conducted a study jointly with SUN Shigang,Professor ofXiamen University and other researchers. They used southern magnolia leaves as precursors,combined with a simple pyrolysis and impurity removal process, and finally obtained biomass-derived hard carbon materials. Thanks to the layered porous structure of the main body, abundant oxygen-containing functional groups on the surface and expanded interlayer spacing of graphite microcrystals, the materials showed high reversible capacity and excellent cycle stability. The reversible specific capacities of SIBs and KIBs anodes were 315 and 263.5 mAh/g, respectively, and good capacity retentioncould be obtained even after 2000 cycles at a high rate of 2000 mAh/g.
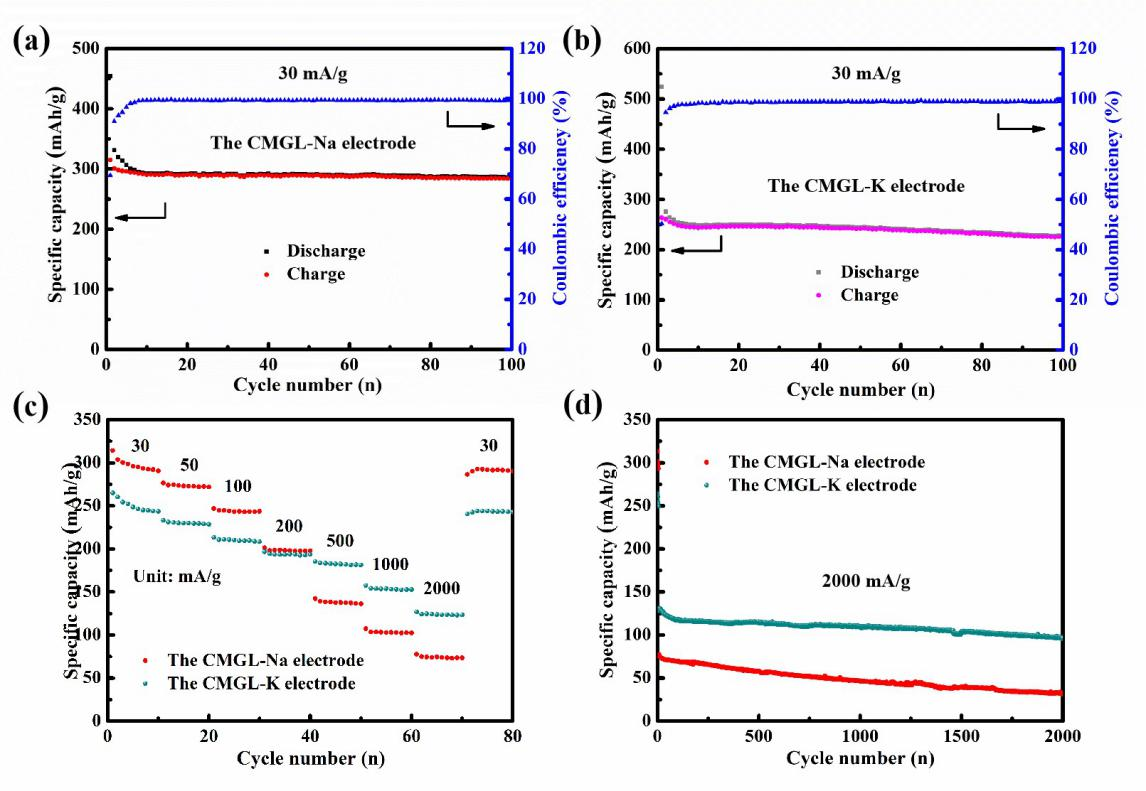
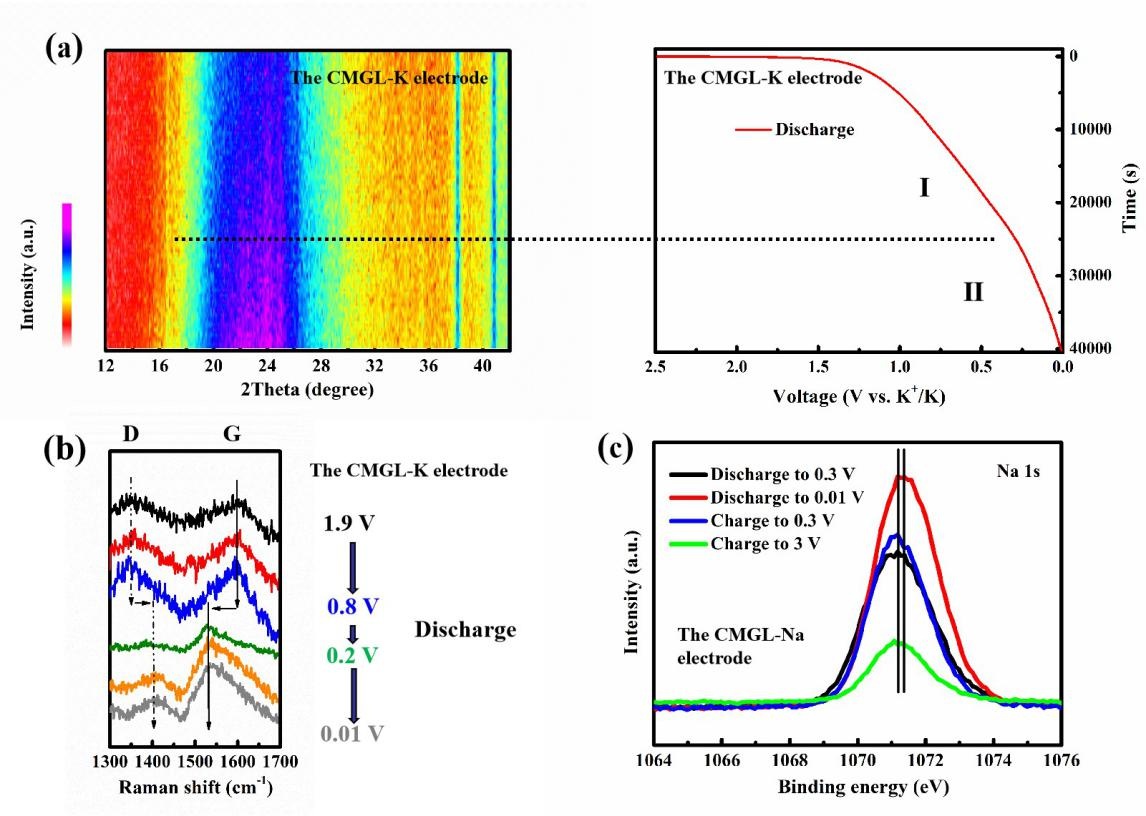
As for the controversial storage mechanism of sodium/potassium, the electrochemical energy storage behavior of materials was further clarified by using galvanostatic intermittent titration (GITT), cyclic voltammetry (CV) with various scanning rates, in situ X-ray diffraction (XRD), in situ Raman spectroscopy and X-ray photoelectron spectroscopy (XPS). The experimental results showed that the high potential slope region corresponds to the adsorption characteristics of the alkali metal ions on the material surface, while the low potential quasi-platform region belongs to the intercalation behavior of the alkali metal ions between graphite microcrystalline layers, that is, the "adsorption-intercalation" model. This work provides a new research direction for designing more high-capacity anode materials.
Recently, the relatedfindings were published as a back-cover article in Carbon Energy, a new high starting point journal in China, with KUST as the first listed university of the paper.
Translated by: YANG Fuyu, Faculty of Foreign Languages and Cultures
Edited by: DUAN Tianting, Faculty of Foreign Languages and Cultures (English)
Source: National and Local Joint Engineering Laboratory for Lithium-Ion Batteries and Materials Fabrication Technology
Issued by: Division ofInternational Cooperation (English)
Edited by: KUST News Center (Chinese)